Why Are IND and NDA Critical in Drug Legislation?
Understanding drug legislation is crucial for stakeholders in the pharmaceutical industry. Key components of this process are the Investigational New Drug (IND) application and the New Drug Application (NDA). These elements serve as keystones in ensuring that new medical therapies are both safe and effective for public use. By delving into the significance of the ind nda processes, we can appreciate their roles in drug development and regulatory compliance, ultimately influencing the availability of innovative treatments.
Importance of the IND and NDA in Drug Legislation
The Role of IND in Drug Development
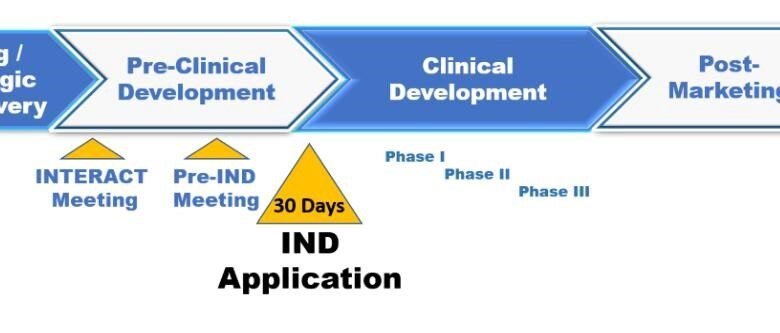
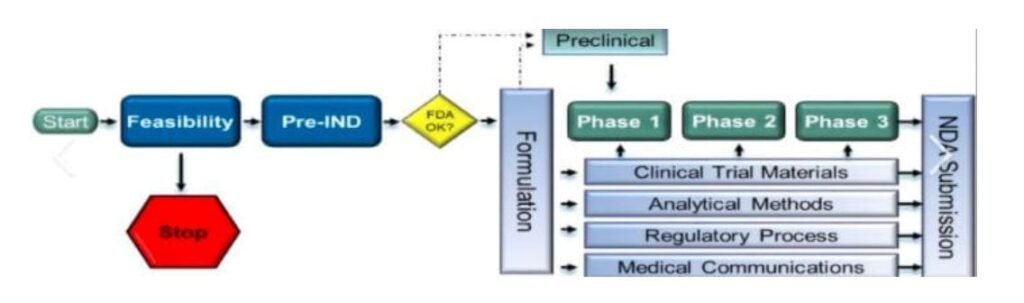
The Investigational New Drug application is a pivotal first step in the clinical trial phase of drug development. Before a new drug can be tested in humans, a company must submit an IND to the U.S. Food and Drug Administration (FDA). This application provides comprehensive data on the drug’s composition, manufacturing protocols, pharmacology, and toxicology. It is scrutinized to ensure that clinical trials do not pose undue risk to participants. The IND is critical because it establishes a framework for how clinical trials should be conducted, safeguarding participants while collecting vital data on the drug’s effects. If the FDA approves the IND, the sponsor can initiate clinical trials. This permission is crucial as it represents a significant step towards bringing a new therapy to the market. Moreover, the approval acts as a quality assurance measure, indicating that the drug has shown potential benefits based on preclinical studies. Thus, the IND not only protects human subjects but also sets the stage for subsequent development stages.
See also: Anavar What Are The Health Benefits Of Anavar 50 mg And How To Use It?
Navigating the NDA Process: Ensuring Drug Safety and Efficacy
Following successful clinical trials, the next major hurdle for a pharmaceutical company is obtaining approval through a New Drug Application. The NDA is a rigorous process that demands a comprehensive dossier demonstrating a drug’s safety and efficacy. This application must include all data from clinical trials, proposed labeling, safety updates, drug abuse information, patient information, and directions for use. Essentially, the NDA seeks to provide enough evidence to convince the FDA that the drug is safe for its intended use. For companies, securing NDA approval is the pinnacle of drug development. It signifies that the drug can be marketed and made available to patients. Inadequate preparation or incomplete submissions can lead to significant delays, impacting both the financial health of the company and patient access to new therapies. Therefore, meticulous attention to detail in preparing the NDA is imperative. This entails robust data collection and management throughout the clinical trials, ensuring that all findings are accurately represented in the submission.
Legal and Regulatory Implications of IND and NDA
Both the IND and NDA processes serve as legal and regulatory checkpoints that help maintain high standards within the pharmaceutical industry. These requirements are not just bureaucratic hurdles; they are legal safeguards designed to protect public health. They ensure that any new drug introduced to the market has undergone comprehensive testing and review, minimizing risks to patients. Legal implications also extend to intellectual property. Through these applications, companies solidify their patent claims and market exclusivity rights, which protect their innovations and incentivize further research and development. Compliance with IND and NDA regulations affirms that a company adheres to federal and international laws governing pharmaceuticals, thereby enhancing its reputation and consumer trust.
Conclusion
IND and NDA processes play an integral role in modern drug legislation, ensuring safe and effective therapies reach the market. They represent complex but necessary pathways that uphold public health standards and facilitate the responsible introduction of new medical solutions. Of course, if you find this article helpful, you may wish to share it with your family and friends.




